 |
|
叶松 研究员
国家杰出青年基金获得者 电话:86-10-62641156; 传真:86-10-62554449 |
学术经历
1991.9-1995.7 华东师范大学 学士
1997.9-2002.7 中国科学院上海有机化学研究所 博士
2002.9-2005.7 美国国家卫生研究院 博士后
2005.7-至今 中国科学院化学研究所 研究员
奖励与荣誉
2002 中国科学院院长特别奖
2004 全国百篇优秀博士学位论文
2012 Thieme Chemistry Journals Award
2012 国家自然科学二等奖(第三完成人)
2014 国家杰出青年基金
2015 "中国科学院"优秀研究生指导教师
研究方向
合成反应是有机合成化学的重要基础,课题组致力于高效、高选择性有机合成方法的发现与应用,发展不对称催化反应,实现重要有机分子骨架的高效快速构建。具体研究方向包括:
1. 氮杂环卡宾催化
2. 光催化协同催化
3. 串联反应
4. 杂环化合物合成
Selected Work
1. N-Heterocyclic carbene (NHC) catalyzed cross-coupling of homoenolate and enolate
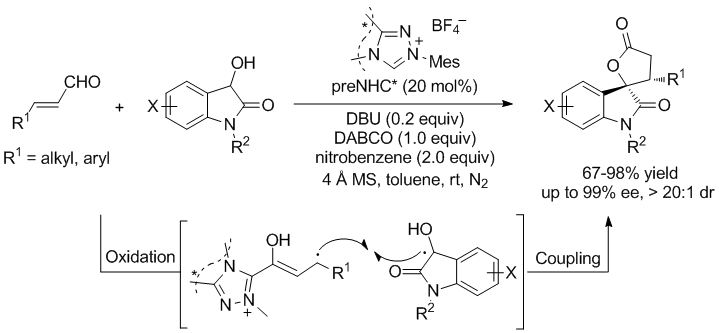
2. NHC-catalyzed [3+4] annualtions

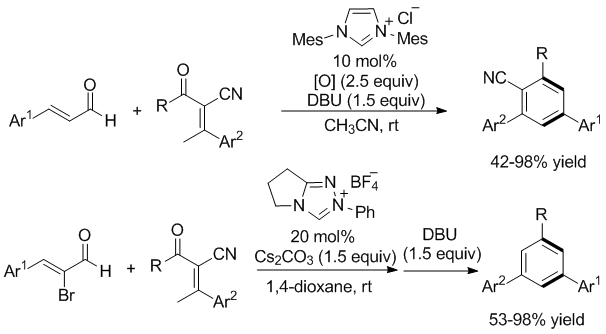
3. NHC-catalyzed Synthesis of multisubstituted benzenes
Selected Publications
1. Chen, X.-Y.; Chen, K.-Q.; Sun, D.-Q.;* Ye, S.* N-Heterocyclic carbene-catalyzed oxidative [3 + 2] annulation of dioxindoles and enals: cross coupling of homoenolate and enolate Chem. Sci., 2017, 8, 1936.
2. Zhang, Z.-F.; Chen, K.-Q.; Zhang, C.-L.; Ye, S.* N-heterocyclic carbene-catalyzed synthesis of spirocyclopentene-oxindoles from bromoenals Chem. Commun., 2017, 53, 4327.
3. Yi, L.; Zhang, Y.; Zhang, Z.-F.; Sun, D.-Q.;* Ye, S.* Synthesis of Dihydropyridinone-Fused Indoles and α‑Carbolines via N‑Heterocyclic Carbene-Catalyzed [3+3] Annulation of Indolin-2-imines and Bromoenals Org. Lett. 2017, 19, 2286.
4. Zhang, C.-L.; Gao, Z.-H.; Liang, Z.-Q.; Ye, S.* N-Heterocyclic Carbene-Catalyzed Synthesis of Multi-Substituted Benzenes from Enals and α-Cyano-β-methylenones Adv. Synth. Catal. 2016, 358, 2862.
5. Zhang, C.-L.; Ye, S.* N‑Heterocyclic Carbene-Catalyzed Construction of 1,3,5-Trisubstituted Benzenes from Bromoenals and α‑Cyano-β-methylenones Org. Lett. 2016, 18, 6408.
6. Liang, Z.-Q.; Gao, Z.-H.; Jia W.-Q.; Ye, S.* “Bifunctional N-Heterocyclic Carbene Catalyzed [3+4] Annulation of Enals and Aurones” Chem. Eur. J. 2015, 21, 1868.
7. Chen, X.-Y.; Gao, Z.-H.; Song, C.-Y.; Zhang, C.-L. Wang, Z.-X.;* Ye, S.* “N-Heterocyclic Carbene Catalyzed Cyclocondensation of α,β-Unsaturated Carboxylic Acids: Enantioselective Synthesis of Pyrrolidinone and Dihydropyridinone Derivatives” Angew. Chem. Int. Ed. 2014, 53, 11611.
8. Chen, X.-Y.; Xia, F.; Cheng, J.-T.; Ye, S.* “Highly Enantioselective γ-Amination by N-Heterocyclic Carbene Catalyzed [4+2] Annulation of Oxidized Enals and Azodicarboxylates” Angew. Chem. Int. Ed. 2013, 52, 10644.
9. Lv, H.; Jia, W.-Q.; Sun, L.-H.; Ye, S.* “N-Heterocyclic carbene catalyzed [4+3] annulation of enals and o-quinone methides: highly enantioselective synthesis of benzo-ɛ-lactones A” Angew. Chem. Int. Ed. 2013, 52, 8607.
10. Shen, L.-T.; Jia, W.-Q.; Ye, S.* “Catalytic [4+2] Cyclization of α,β-Unsaturated Acyl Chlorides with 3-Alkylenyloxindoles: Highly Diastereo- and Enantioselective Synthesis of Spirocarbocyclic Oxindoles” Angew. Chem. Int. Ed. 2013, 52, 585.
11. Shen, L.-T.; Sun, L.-H.; Ye, S.* “Highly enantioselective γ-amination of α,β-unsaturated acyl chlorides with azodicarboxylates: Efficient synthesis of chiral γ-amino acid derivatives” J. Am. Chem. Soc. 2011, 133, 15894.
12. Shao, P.-L.; Chen, X.-Y.; Ye, S.* “Formal [3+2] cycloaddition of ketenes and oxaziridines catalyzed by chiral Lewis bases: Enantioselective synthesis of oxazolin-4-ones” Angew. Chem. Int. Ed. 2010, 49, 8412.
13. Huang, X.-L.; He, L.; Shao, P.-L.; Ye, S.* “[4+2] Cycloaddition of ketenes with N-benzoyldiazenes catalyzed by N-heterocyclic carbenes” Angew. Chem. Int. Ed. 2009, 48, 192.
14. Zhang, Y.-R.; He, L.; Wu, X.; Shao, P.-L.; Ye, S.* “Chiral N-heterocyclic carbene catalyzed staudinger reaction of ketenes with imines: Highly enantioselective synthesis of N-Boc β-Lactams” Org. Lett. 2008, 10, 277.

